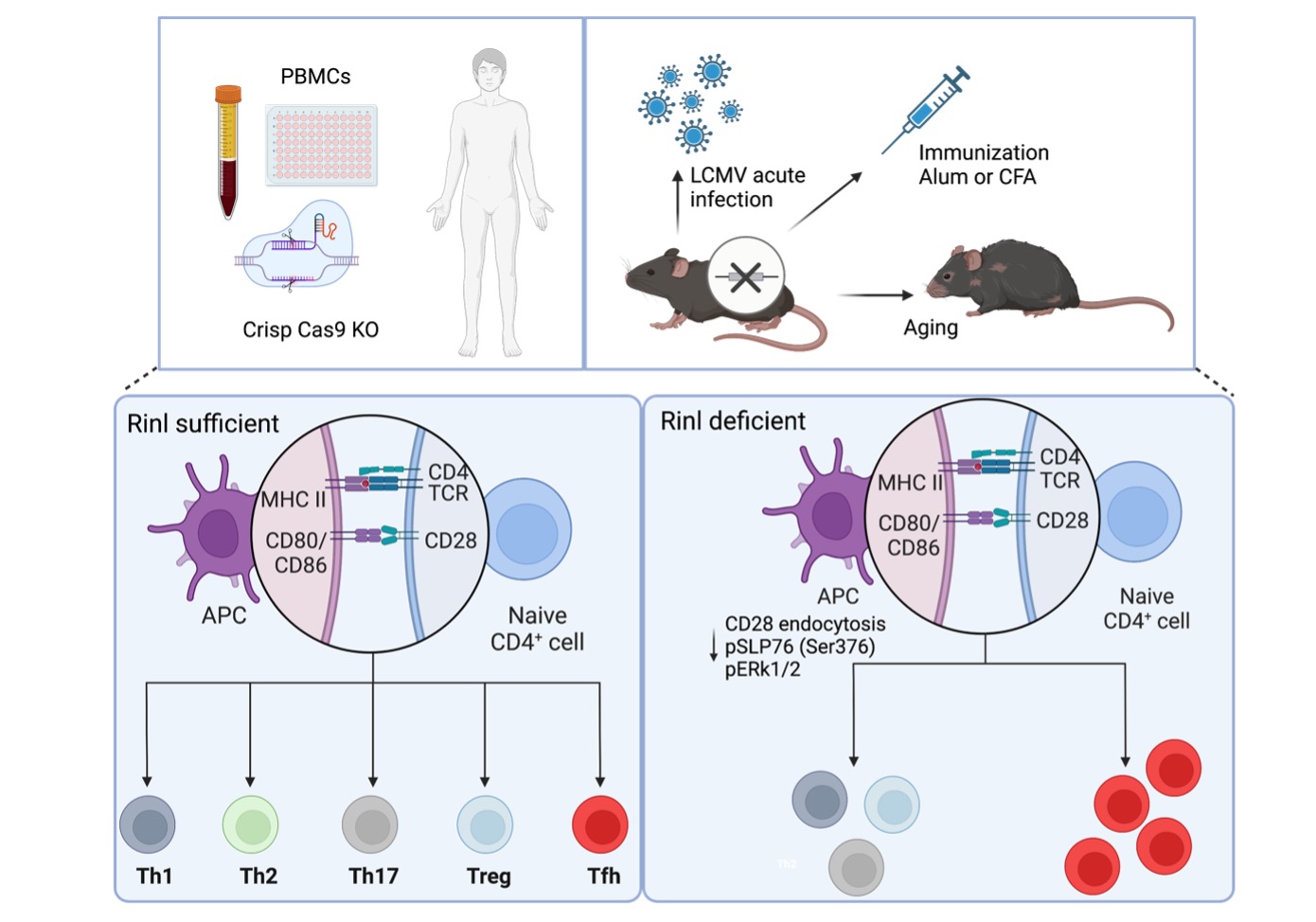
Molecular determinants for the differentiation of murine and human naïve CD4+ T cells into T follicular helper cells and T cell help for B cell maturation and antibody class-switching.
Tfh cells communicate with their environment via so-called receptors. These are proteins that are located on the cell surface, perceive signals from the environment and pass them on to the inside of the cell. In this way, cellular processes are controlled. Cellular processes include cell division and proliferation or the release of new messenger substances, which can then be perceived by other cells via receptors. Therefore, the regulation and fine-tuning of the surface expression of receptors is an important checkpoint to control the communication between cells and their environment. One of these regulatory steps occurs through the so-called "endocytosis process", which causes receptors to be removed from the cell surface and sent inside the cell. Thereby new signaling pathways can occur within the cell, leading to new cellular processes and fates.
A family of proteins that control endocytic processes is formed by the RIN proteins (Rin1, Rin2, Rin3 and Rin-like). Rin-like (Rinl) was discovered in the lab of Ruth Herbst. In our recent study, we were able to show that Rinl regulates endocytosis of a receptor that is important for T cells, the CD28 co-receptor. Altered CD28 endocytosis was associated with changes in the signaling pathways emanating from the CD28 co-receptor, ultimately specifically impacting on the development of Tfh cells. We were able to show that Rinl is an important restrainer of Tfh development, both during immunization, viral infections or aging. Thus, we were able to uncover new mechanisms regulating Tfh differentiation with consequences on human disease like rheumatoid arthritis or lymphoma.
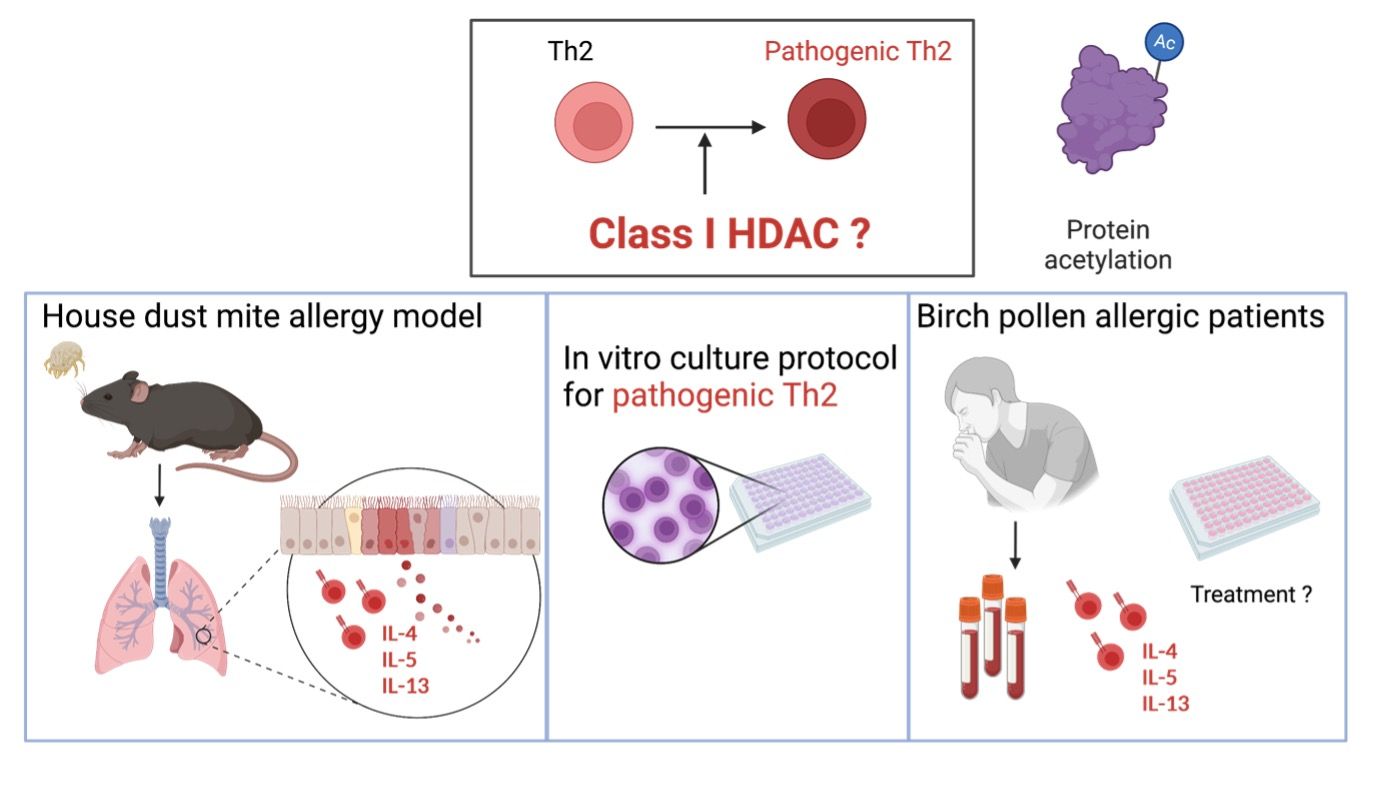
Tissue adaptation of Th2 effector cells during allergic airway disease, with a particular interest how class I HDAC inhibition is regulating this process
Lung pathogenic T helper type 2 (pTh2) cells have been identified as the main drivers of allergic asthma, but fundamental questions remain regarding their heterogeneity, signatures, and molecular regulation. A major limitation to our understanding of the molecular mechanisms regulating pTh2 cells is the lack of an appropriate system to generate and investigate them in vitro. It is well known that the differentiation and effector functions of immune cells are tightly regulated by epigenetic processes such as reversible lysine acetylation orchestrated by histone acetyltransferases and histone deacetylases (HDACs). HDAC1, a class I histone deacetylase, is an important epigenetic regulator of T cells, but its role in pTh2 cells is yet to be determined. Here, using a house dust mite model of allergic asthma, single-cell RNA sequencing (scRNA-seq) of lung CD4+ T cells, and mice with a T cell-specific deletion of HDAC1, we uncover the heterogeneity and signatures of murine pTh2 cells in allergic asthma, and the role of HDAC1 in these cells. Our analyses reveal two distinct subsets of lung pTh2 cells: pathogenic effector Th2 (peTh2) and Th2 tissue-resident memory (Th2 Trm) cells. Both pTh2 cell subsets are highly proinflammatory and exhibit distinct transcriptional and phenotypic signatures as compared with other lung Th subsets. From our scRNA-seq analysis, we identify conditions to generate pTh2 cells in vitro and confirm that these in vitro generated cells have a similar transcriptional profile as lung peTh2 cells.
Methods used: animal disease models, multi-color-flow-cytometry, mouse genetic approaches, advanced primary cell culture techniques (human and mouse CD4+ T cell cultures), proteomic and high-throughput sequencing.
Collaborators
Dirk Baumjohan
Christoph Bock
Barbara Bohle
Michael Bonelli
Clarissa Campbell
Wilfried Ellmeier
Michelle Epstein
Iris Gratz
Markus Hartl
Johan Henriksson
Ruth Herbst
Matteo Iannacone
Karl Kuchler
Mirela Kuka
Thomas Krausgruber
Shinya Sakaguchi
Klaus Schmetterer
Michael Trauner